 |

| We are now UF Health Cancer Center
UF Shands Cancer Center is now UF Health Cancer Center. The new “umbrella” term reflects the health system’s strong ties to UF, a key part of what differentiates the academic health center, with its focus on excellence in research, teaching and patient care, from its competitors. Broad name recognition will help attract and retain the most talented physicians, nurses, faculty, staff and students as well as secure research funding. That translates to better care, better health and better outcomes for patients. Read more |
|
|
Oncology Kudos |
In The News|
Clinical Trials
|
|
June 2013 • Vol. 3 • Issue 2
Oncology Kudos

|
| Sadasivan Vidyasagar, M.D., Ph.D. |
|
Research grant awarded
The National Institutes of Health recently awarded the Molecular Mechanisms of Intestinal Metal Ion Transport During Iron Deficiency project an R01 grant. Sadasivan Vidyasagar, M.D., Ph.D., is the co-principal investigator of the project, with James Collins, Ph.D., of the Institute of Food and Agricultural Sciences, serving as the principal investigator. The project funding will last from March 2013 to February 2017 and will total $325,707. |

|
| Yehia Daaka, Ph.D. |
|
Cancer researcher to lead department of anatomy and cell biology
Yehia Daaka, Ph.D., has been appointed chair of the department of anatomy and cell biology at the University of Florida College of Medicine. Daaka is currently the scientific director of the UF Prostate Disease Center and the David A. Cofrin Chair in Urologic Oncology in the department of urology, as well as a professor of urology and anatomy and cell biology. He began his new role as chair on June 10.
Read more |
|
|
Abstracts selected for ASTRO oral presentation
Twenty seven abstracts have been selected for oral presentation at the Annual Meeting Program Committee of the American Society for Radiation Oncology. The 2013 Annual Meeting will be held Sept. 22-25 in Atlanta, Ga.
Click here for full list |
In The News

|
| John Wingard, M.D. |
|
LifeSouth receives FDA license
LifeSouth has been approved by the Food and Drug Administration for cord blood manufacturing. LifeCord, which is a program of LifeSouth, became only the fifth public cord blood bank in the country to receive its FDA license, and the first in Florida, Georgia and Alabama where it currently operates. Umbilical cord blood contains the stem cells that can be used by patients needing marrow transplants for the treatment of leukemia and other serious diseases. John Wingard, M.D., Price Eminent Scholar and a UF professor of medicine, UF Health Cancer Center deputy director for research, director of the UF Health Bone Marrow Transplant Program and LifeCord's medical director, first proposed starting a public cord blood bank in North Florida in 1996.
Read more
|

|
| Mansour Mohamadzadeh, Ph.D. |
|
New UF center focuses on inflammation and disease
Recognizing growing evidence that inflammation influences many diseases, University of Florida Health has established the Center for Inflammation and Mucosal Immunology to foster collaboration among members of the UF biomedical research community with shared interest in inflammation and disease. The center hosts a monthly seminar series wherein members share their research and learn about the research of others. In addition, access to core equipment and animal research platforms, such as germ-free zebrafish and mouse models, are benefits of membership.
Read more
|
|
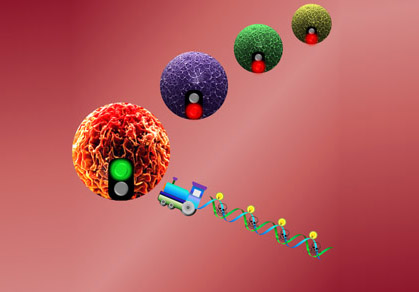
| "DNA Nanotrain" |
|
UF researchers develop 'nanotrain' for targeted cancer drug transport
The lab of Weihong Tan, Ph.D., a UF distinguished professor of chemistry, and professor of physiology and functional genomics, has developed an organic, three-dimensional DNA nanostructure that precisely targets cancer cells. The report, published in a recent issue of the Proceedings of the National Academy of Sciences, describes Tan’s “DNA nanotrain” as being composed of short strands of DNA tethered together into one long train. On the end of the nanotrain is an aptamer, a tiny piece of nucleic acid serving as the train’s “locomotive” on biochemical autopilot to hone in on and bind to specific cancer cells.
Read more |
Clinical Trials
A Phase II Clinical Trial of Lenalidomide and Prednisone in Low and Intermediate-1 IPSS Risk, non-del (5q) MDS Patients
The purpose of this research is to evaluate the use of lenalidomide and prednisone in people with Myelodysplastic Syndrome (MDS).
Lenalidomide is a drug that alters the immune system and it may also interfere with the development of tiny blood vessels that help support tumor growth. Therefore, in theory, it may reduce or prevent the growth of cancer cells. Lenalidomide is approved by the U.S. Food and Drug Administration (FDA) for the treatment of specific types of myelodysplastic syndrome (MDS) and in combination with dexamethasone for people with multiple myeloma (MM) who have received at least 1 prior therapy. MDS and MM are cancers of the blood. It is currently being tested in a variety of cancer conditions. As it is being used in this study it is considered an investigational use. An "investigational use" is a use that is being tested and is not approved by the FDA.
Prednisone is approved by the FDA to treat numerous conditions. In addition, prednisone is approved by the FDA to treat Low or Intermediate-1 IPSS Risk, non-del (5q) MDS. Protocol No. MCC-16099
Principal Investigator:
Christopher Cogle, M.D.
|
A Randomized Phase II Study of Azacitidine in Combination with Lenalidomide (NSC-703813) vs. Azacitidine Alone vs. Azacitidine in Combination with Vorinostat (NSC-701852) for Higher-Risk Myelodysplastic Syndromes (MDS)
This randomized phase II trial studies how well giving azacitidine works with or without lenalidomide or vorinostat in treating patients with higher-risk myelodysplastic syndromes or chronic myelomonocytic leukemia. Drugs used in chemotherapy, such as azacitidine, work in different ways to stop the growth of cancer cells, either by killing the cells or stopping them from dividing. Lenalidomide may stop the growth of cancer cells by stopping blood flow to the cancer. Vorinostat may stop the growth of cancer cells by blocking some of the enzymes needed for cell growth. It is not yet known whether azacitidine is more effective with or without lenalidomide or vorinostat in treating myelodysplastic syndromes or chronic myelomonocytic leukemia. Protocol No. S1117
Principal Investigator:
Jack Hsu, M.D.
|
NSABP Patient Registry and Biospecimen Profiling Repository (MPR-1)
Primary Outcome Measures:
To characterize common genetic/molecular profiles associated with colorectal cancer (CRC)
Secondary Outcome Measures:
To identify subpopulations of registry patients on the basis of their molecular profiles that may be eligible for participation in available NSABP clinical trials involving novel agents
To conduct further analysis of tumor samples for the discovery of new potential targets and mechanisms of drug resistance. Protocol No. MPR-1
Principal Investigator: Thomas George, M.D. |
A Phase I Window, Dose Escalating and Safety Trial of Metformin in Combination with Induction Chemotherapy in Relapsed Refractory Acute Lymphoblastic Leukemia: Metformin with Induction Chemotherapy of Vincristine, Dexamethasone, Doxorubicin, and PEG-asparaginase (VPLD)
1. To demonstrate the safety and feasibility of the addition of Metformin to induction chemotherapy for recurrent ALL.
2. To demonstrate the maximum tolerated dose (MTD) of metformin when given twice daily by mouth to children receiving induction chemotherapy and describe the pharmacokinetics of metformin when given in this setting. Protocol No. MCC-16601
Principal Investigator: William Slayton, M.D. |
A Phase III Randomized Study of Adjuvant Ipilimumab Anti-CTLA4 Therapy Versus High-Dose Interferon á-2b for Resected High-Risk Melanoma
First Co-primary Endpoint: To evaluate recurrence-free survival (RFS) between patients randomized to receive post-operative adjuvant pilimumab versus those randomized to receive HDI.
Second Co-primary Endpoint: To evaluate overall survival (OS) between patients randomized to receive post-operative adjuvant ipilimumab versus those randomized to receive HDI.
Secondary Endpoints
To evaluate safety and tolerability of post-operative adjuvant ipilimumab therapy.
Among patients enrolled by CCOPs, to compare the global QOL between two treatments using FACT-G form and to evaluate the effect of treatment-related side effects that may have an impact on the health-related domains of QOL using FACITD and FACT-BRM. Protocol No. E1609
Principal Investigator: Stephen Staal, M.D. |
A Phase I Trial of Dose Escalation of Metformin in Combination with Vincristine, Irinotecan, and Temozolomide in Children with Relapsed or Refractory Solid Tumors
1.1.1 To determine the maximum tolerated dose (MTD) of metformin when
given in conjunction with VIT in children with refractory and relapsed
solid tumors.
1.1.2 To describe the pharmacokinetics of metformin in children with relapsed
malignancies receiving VIT combination chemotherapy. Protocol No. MCC-16962
Principal Investigator: Joanne Lagmay, M.D. |
|
Contact Us
Do you have a great story, or have you been honored with an award or had research accepted for publication? Let us know! We'll include it in the Cancer Connection eNews, which is distributed monthly to UF Health Cancer Center members and the faculty and staff of the center's multidisciplinary cancer programs during the fall and spring semesters. Send your news to:
Lindy Brounley
Communications Director
UF Health Cancer Center
Phone: 352-273-8013
E-mail: brounley@ufl.edu

|
|
|